Graphene Jolts Sodium-Ion Battery Capacity
Soon after yrs of anticipation, sodium-ion batteries are commencing to provide on their guarantee for strength storage. But so considerably, their commercialization is limited to big-scale employs these as storing strength on the grid. Sodium-ion batteries just will not have the oomph essential for EVs and laptops. At about 285 Wh/kg, lithium-ion batteries have 2 times the strength density of sodium, creating them extra suited for individuals transportable applications.
Researchers now report a new style of graphene electrode that could improve the storage potential of sodium batteries to rival lithium’s. The substance can pack approximately as several sodium ions by quantity as a common graphite electrode does lithium. It opens up a path to creating reduced-price tag, compact sodium batteries practical.
Plentiful and inexpensive, and with comparable chemical homes as lithium, sodium is a promising substitution for lithium in up coming-technology batteries. The security and safety of sodium batteries helps make them in particular promising for electronics and cars and trucks, where by overheated lithium-ion batteries have in some cases established dangerous.
“But at the moment the important challenge with sodium-ion batteries is that we will not have a suited anode substance,” states Jinhua Solar, a researcher in the department of industrial and components science at Chalmers University of Technologies.
For the battery to cost swiftly and shop a lot of strength, ions have to have to simply slip in and out of the anode substance. Sodium-ion batteries use cathodes built of sodium metal oxides, though their anodes are normally carbon-primarily based anodes just like their lithium cousins even though Santa Clara, California-primarily based Natron Electricity is creating both its anodes and cathodes out of Prussian Blue pigment utilized in dyes and paints.
Some sodium battery developers are applying activated carbon for the anode, which retains sodium ions in its pores. “But you have to have to use high-grade activated carbon, which is quite high priced and not uncomplicated to make,” Solar states.
Graphite, which is the anode substance in lithium-ion batteries, is a decrease price tag option. Having said that, sodium ions do not transfer effectively in between the stack of graphene sheets that make up graphite. Researchers utilized to imagine this was mainly because sodium ions are greater than lithium ions, but turns out even-greater potassium ions can transfer in and out simply in graphite, Solar states. “Now we imagine it’s the surface area chemistry of graphene layers and the electronic structure that are not able to accommodate sodium ions.”
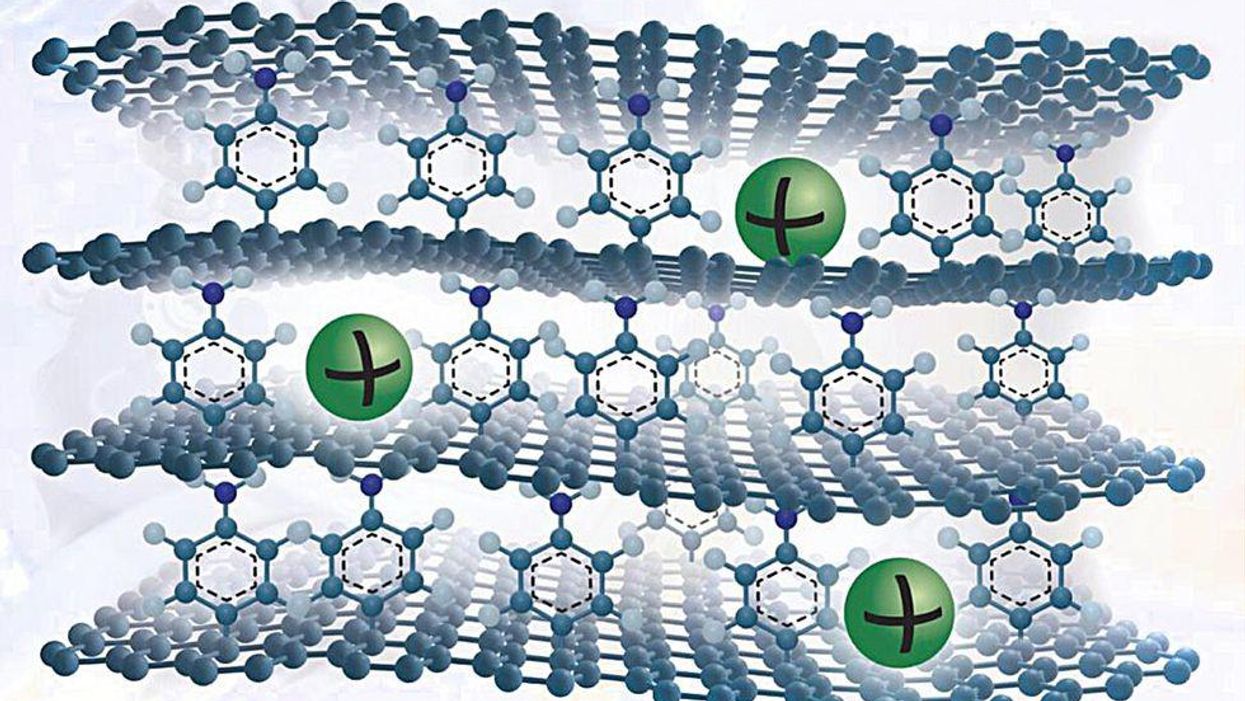
He and his colleagues have arrive up with a new graphite-like substance that overcomes these problems. To make it, they mature a solitary sheet of graphene on copper foil and attach a solitary layer of benzene molecules to its major surface area. They mature several these graphene sheets and stack them to make a layer cake of graphene held apart by benzene molecules.
The benzene layer improves the spacing in between the layers to let sodium ions to enter and exit simply. They also develop defects on the graphene surface area that as as lively reaction sites to adsorb the ions. Furthermore, benzene has chemical teams that bind strongly with sodium ions.
This seemingly basic technique boosts the material’s sodium ion-storing potential significantly. The researchers’ calculations show that the potential matches that of graphite’s potential for lithium. Graphite’s potential for sodium ions is normally about 35 milliAmpere-hours for every gram, but the new substance can maintain about 330 mAh/g, about the same as graphite’s lithium-storing potential.







