A Lithium-Ion Battery That Works Even When It’s on Fire
In a paper released very last thirty day period in Nano Letters, the workforce describe how they’ve developed a novel “fireproof” strong-point out electrolyte (SSE) for use in lithium-ion batteries. “We handle the difficulty of flammability in SSEs by introducing a fireplace retardant,” states Jiayu Wan, a postdoctoral researcher in Cui’s lab and co-creator of the paper.
They employed a flame-retardant substance known as decabromodiphenyl ethane, or DBDPE for brief. To make their new strong-point out electrolyte, the workforce to start with developed a slim movie by combining DBDPE with polyimide, a mechanical enforcer.
Using polyimide has lots of benefits, states Wan. Aside from currently being “mechanically seriously robust,” it boasts a superior melting point (generating it considerably less most likely that a brief circuit will come about), a solutions-dependent producing process (that’s compatible with how batteries are built nowadays), and it is economical (3M even has movie tapes built from it).
The hitch, having said that, is that polyimide cannot perform ions. To get all-around this snag, Wan and his colleagues extra two distinctive polymers, polyethylene oxide (PEO) and lithium bistrifluoromethanesulfonylimide (LiTFSI), to the combine.
“It’s innovative—they’ve neatly employed co-polymers, which is a new way to remedy the flammable polymer electrolyte battery difficulty,” states Chunsheng Wang, a researcher who scientific studies new battery technologies at the University of Maryland.
Good-point out electrolytes just take two key sorts. You can make them from ceramics, a substance that conducts ions properly but is extremely brittle and effects in thick batteries, which have reduce electrical power density. Or, you can have electrolytes composed of polymers, which are minimal cost, lightweight, and versatile. They are also “soft,” which means there’s minimal resistance alongside the interface of the electrode and electrolyte, which lets the electrolyte to perform ions simply.
But polymer electrolytes also have issues. “This softness suggests they’re not able to suppress lithium dendrite propagation, so they’re flammable,” states Wang, referring to the little needle-like projections that grow from a battery’s anode. Dendrites can consequence just after recurring cycles of charging and discharging when these lithium crystals pierce a battery’s separator, they can begin fires.
“A lot of men and women believe that that for liquid electrolytes, there is no resistance and dendrites can grow by the electrolyte,” states Wang. “But if you replace the liquid with a strong, which is mechanically more powerful, the lithium may possibly be blocked.”
Their mechanical toughness, alongside with minimized flammability, are just some good reasons why strong-point out electrolytes have garnered fascination amid researchers in equally academia and market. A 3rd motive lies with the point that they make it possible for batteries to be stacked. “Because the electrolyte does not movement, you can simply place them collectively without the need of wires… which is vital for expanding electrical power density,” states Wang.
There is no great choice, even though. “All the distinctive SSEs have some difficulties, so you have to balance them out,” he states.
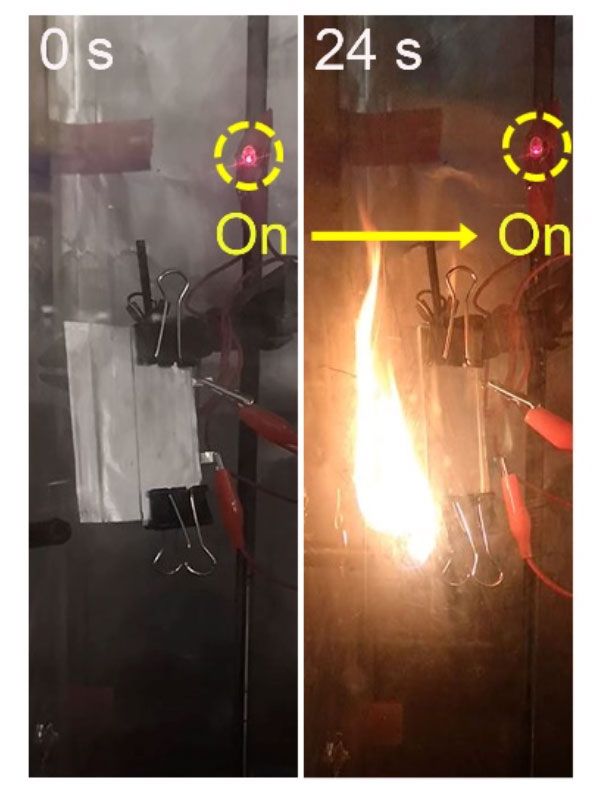
It is a target that the workforce at Stanford looks a person step closer to reaching. Not only is their new strong-point out electrolyte ultrathin (measuring among ten to 25 micrometers), it also delivers a superior particular capability (131 milliampere hours for every gram, mAh/g, at 1 diploma C), and demonstrates good biking performance (long lasting 300 cycles at sixty levels C). Crucially, prototype battery cells built employing it proved to perform inspite of catching fireplace (in this movie, an LED remains lit even even though the battery powering it is on fireplace).
“This was extremely stunning to us,” states Stanford’s Wan. “Usually a battery will just explode with a fireplace. But with this a person, not only does it not explode, it even now capabilities.”
These days, the workforce proceeds to investigate new components and buildings for use in strong-point out electrolytes, with the goal of enhancing current density and mobile capability. Says Wan: “The problem now is to make the battery demand a lot quicker, have a increased electrical power density, and to very last more time.”






